R&D: Rigid Bonds Enabling New Storage Technology
Study explains how switching process in promising new technology based on glass formation can be fast and reliable at same time.
This is a Press Release edited by StorageNewsletter.com on June 20, 2019 at 2:30 pmFrom European XFEL GmbH
Experiments with X-ray lasers show how new phase-change materials could lead to more efficient and faster data storage technologies.
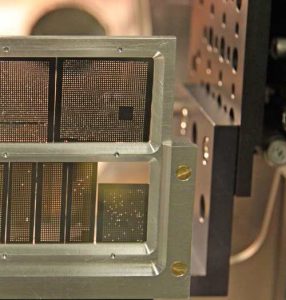
European XFEL scientist Peter Zalden (left) and colleagues at the FXE instrument at European XFEL. In a paper published today in Science, a group of scientists led by Zalden and researchers from the University of Duisburg-Essen in Germany, describe how they used the X-ray free-electron laser LCLS at the SLAC National Accelerator Laboratory, California, to show that a transition in the chemical bonding mechanism enables the data storage in phase-change materials used in smartphones. The results can be used to optimize phase-change materials for faster and more effective data storage technologies. Similar experiments are already scheduled at the instrument for Femtosecond X-ray Experiments (FXE) at European XFEL, where the X-ray pulses are short and intense enough to capture snapshots of these fast processes.
(Copyright: European XFEL/Jan Hosan)
Phase-change materials are used in the latest generation of smartphones enabling higher storage densities and energy efficiency. Data is recorded by switching between glassy and crystalline material states by applying a heat pulse. However, to date it has not been possible to study what happens at the atomic level during this process. In a paper published today in Science, a group of scientists led by researchers from European XFEL and the University of Duisburg-Essen in Germany, describe how they used the capabilities of the X-ray free-electron laser LCLS at the SLAC National Accelerator Laboratory, California, USA, to show that a transition in the chemical bonding mechanism enables the data storage in these materials. The results can be used to optimize phase-change materials for faster and more effective data storage technologies. They also provide new insights into the process of glass formation.
Phase-change materials made of compositions of the elements antimony, tellurium and germanium, can be used to store increasingly large amounts of data, and do so quickly and energy efficiently. They are used, for example, in replacements for flash drives in the latest generation of smartphones. When an electrical or optical pulse is applied to heat these materials locally, they change from a glassy to a crystalline state, and vice versa. These two different states represent the ‘0’ and ‘1’ of the binary code needed to store information. However, to date it has not been possible to resolve how exactly these changes of state occur on an atomic level.
In an experiment at the Linac Coherent Light Source (LCLS) in California, a group of scientists led by researchers from European XFEL and the University of Duisburg-Essen used a technique called femtosecond X-ray diffraction to study atomic changes when the materials switch state. In the experiment, that took place before European XFEL was operational, an optical laser was first used to trigger the material to change between crystalline and glassy states. During this extremely fast process, the X-ray laser was used to take images of the atomic structure. Only X-ray Free-Electron Lasers such as LCLS or European XFEL produce pulses that are short and intense enough to capture snapshots of the atomic changes occurring on such short timeframes. The scientists collected over 10,000 images in order to shed light on the sequence of atomic changes that occur during the process.
The sample wafers used for the measurements are arrays of silicon nitride membranes, coated with the active phase-change material. First, the optical laser is absorbed in the active material, followed by an X-ray pulse that transmits and scatters on it, providing information about the atomic structure. A fresh position is used for every switching event to avoid sample deterioration due to the intense X-ray pulses.
(Credit: Klaus Sokolowski-Tinten, University of Duisburg-Essen)
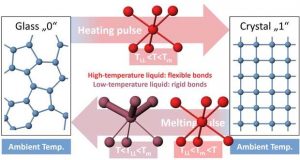
To store information with phase-change materials, they must be cooled quickly to enter a glassy state without crystallizing. They must also stay in this glassy state for as long as the data is to be stored. This means that the crystallization process must be very slow to the point of being almost absent, such as is the case in ordinary glass. At high temperatures, however, the same material must be able to crystallize very quickly to erase the information. The way a material can form a stable glass but at the same time becomes very unstable at elevated temperatures has puzzled researchers for decades.
In their experiment, the researchers studied the fast cooling process, by which a glass is formed. They found that when the liquid is cooled sufficiently far below the melting temperature, it undergoes a structural change to form another, low-temperature liquid. This low-temperature liquid can only be observed on very short timescales, before crystallization takes place. The two different liquids had not only very different atomic structures, but also different behaviors: The liquid at high temperature has a high atomic mobility that enables the atoms to crystallize, i.e., to arrange in a well-ordered structure. However, when the liquid passes below a certain temperature below the melting point, some chemical bonds become stronger and more rigid and can hold the disordered atomic structure of the glass in place. These observations were supported by computer simulations performed at RWTH Aachen University.
The simulations provided further details of the atomic structure of the two liquids and revealed changes in the electronic properties, showing that the high-temperature liquid is more metallic than the low-temperature liquid – consistent with the formation of more rigid bonds. It is only the rigid nature of these chemical bonds that prevents the transformation and – in case of phase-change memory devices – secures the information in place.
The switching cycle in phase-change memory devices is based on two pulses: One to heat the glass to a high temperature, where crystallization proceeds rapidly and another to melt the material, stabilizing the disordered atomic structure of the liquid upon cooling. The liquid-liquid phase transition occurs below the melting point of the material and allows the high-temperature liquid to have a high atomic mobility enabling rapid crystallization over a wide temperature range, whereas the low-temperature liquid has more rigid bonds that can stabilize its disordered atomic structure against crystallization. Therefore, the liquid-liquid phase transition enables fast and reliable data storage in phase-change memory devices.
(Credit: Peter Zalden, European XFEL)
Peter Zalden, scientist, European XFEL and first author of the study explains: “Current data storage technology has reached a scaling limit so that new concepts are required to store the amounts of data that we will produce in the future. Our study explains how the switching process in a promising new technology based on glass formation can be fast and reliable at the same time.”
Klaus Sokolowski-Tinten, University of Duisburg-Essen, who initiated the project, adds: “The results and our time-domain approach can also help to understand how liquids of other classes of materials behave when they are rapidly cooled to temperatures well below the melting point and form a glass. This year we will do similar experiments at different facilities worldwide – including the European XFEL – where the femtosecond pulses are short and intense enough to capture snapshots of these fast processes.”
Team of researchers after performing the experiment at SLAC.
(Credit: Klaus Sokolowski-Tinten, University of Duisburg – Essen)
Click to enlarge
The study, coordinated by European XFEL and University of Duisburg – Essen, was part of an international collaboration including scientists from Forschungszentrum Jülich, Institut Laue-Langevin, Lawrence Livermore National Laboratory, Lund University, Paul Scherrer Institute, SLAC National Accelerator Laboratory, Stanford University, The Spanish National Research Council (CSIC), RWTH Aachen University, and the University of Potsdam.
Article: Femtosecond x-ray diffraction reveals a liquid–liquid phase transition in phase-change materials
Science Magazine has published an article written by Peter Zalden, Stanford PULSE Institute, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, European XFEL, Holzkoppel 4, 22869 Schenefeld, Germany, Florian Quirin, Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Lotharstrasse 1, 47048 Duisburg, Germany, Mathias Schumacher, Institut für Theoretische Festkörperphysik, JARA-FIT and JARA-HPC, RWTH Aachen University, Germany, Jan Siegel,Instituto de Optica, CSIC, C/Serrano 121, 28006 Madrid, Spain, Shuai Wei, I. Physikalisches Institut and JARA-FIT, RWTH Aachen, Sommerfeldstrasse 14, 52074 Aachen, Germany, Azize Koc, Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Lotharstrasse 1, 47048 Duisburg, Germany, and Institut für Physik und Astronomie, Universität Potsdam, Karl-Liebknecht-Strasse 24-25, 14476 Potsdam, Germany, Matthieu Nicoul, Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Lotharstrasse 1, 47048 Duisburg, Germany, Mariano Trigo1,Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Pererik Andreasson, Henrik Enquist, Department of Physics, Lund University, Professorsgatan 1, 223 62 Lund, Sweden,Michael J. Shu, Department of Applied Physics, Stanford University, Stanford, CA 94305, USA, Tommaso Pardini, Lawrence Livermore National Laboratory, Livermore, CA 94550, USA, Matthieu Chollet, Linac Coherent Light Source, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Diling Zhu, Linac Coherent Light Source, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Henrik Lemke, Linac Coherent Light Source, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA and Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen, Switzerland, Ider Ronneberger, Institut für Theoretische Festkörperphysik, JARA-FIT and JARA-HPC, RWTH Aachen University, Germany, Jörgen Larsson,Department of Physics, Lund University, Professorsgatan 1, 223 62 Lund, Sweden, Aaron M. Lindenberg,Stanford PULSE Institute, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, and Department of Materials Science and Engineering, Stanford University, Stanford, CA 94305, USA, Henry E. Fischer,Institut Laue-Langevin, 71 Avenue des Martyrs, CS 20156, 38042 Grenoble Cedex 9, France, Stefan Hau-Riege, Lawrence Livermore National Laboratory, Livermore, CA 94550, USA, David A. Reis1,Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Rd., Menlo Park, CA 94025, USA, Riccardo Mazzarello,Institut für Theoretische Festkörperphysik, JARA-FIT and JARA-HPC, RWTH Aachen University, Germany, Matthias Wuttig, I. Physikalisches Institut and JARA-FIT, RWTH Aachen, Sommerfeldstrasse 14, 52074 Aachen, Germany, and PGI 10 (Green IT), Forschungszentrum Jülich, 52428 Jülich, Germany, and Klaus Sokolowski-Tinten, Faculty of Physics and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, Lotharstrasse 1, 47048 Duisburg, Germany.
Abstract: ”In phase-change memory devices, a material is cycled between glassy and crystalline states. The highly temperature-dependent kinetics of its crystallization process enables application in memory technology, but the transition has not been resolved on an atomic scale. Using femtosecond x-ray diffraction and ab initio computer simulations, we determined the time-dependent pair-correlation function of phase-change materials throughout the melt-quenching and crystallization process. We found a liquid–liquid phase transition in the phase-change materials Ag4In3Sb67Te26 and Ge15Sb85 at 660 and 610 kelvin, respectively. The transition is predominantly caused by the onset of Peierls distortions, the amplitude of which correlates with an increase of the apparent activation energy of diffusivity. This reveals a relationship between atomic structure and kinetics, enabling a systematic optimization of the memory-switching kinetics.”















 Subscribe to our free daily newsletter
Subscribe to our free daily newsletter

