R&D: Detecting Magnetism of Single Atom’s Nucleus From IBM Research
Detected magnetism of single atom’s nucleus, feat that opens door to using nucleus as way to sense and control magnetism at the atomic scale.
This is a Press Release edited by StorageNewsletter.com on November 12, 2018 at 2:25 pmPosted in: IBM Research-Almaden, Nanotechnology
Our team at IBM Research − Almaden in Silicon Valley has detected the magnetism of a single atom’s nucleus, a feat that opens the door to using the nucleus as a way to sense and control magnetism at the atomic scale.
This breakthrough, recently published in the journal Science, was achieved by measuring the magnetic effect of the nucleus on the electrons in the same atom. The study reveals information about the isotope – the number of neutrons in an atom’s nucleus – and how the atom’s magnetization depends on its neighboring atoms, providing a powerful new tool for sensing at the nanoscale and presenting a major step toward using the nucleus for future spintronics.
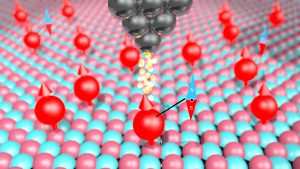
Figure 1: Sketch of the experiment. Each red ball represents a magnetic atom bonded to a surface. Some naturally have a nuclear spin, a small magnet, in their core. The sharp tip of a STM probes a single magnetic atom.
(Image courtesy QNS)
Working with an international team of collaborators including the Center for Quantum Nanoscience (QNS), the University of Oxford and the International Iberian Nanotechnology Laboratory, we measured iron and titanium atoms that were attached to a carefully prepared surface. We used a scanning tunneling microscope (STM), the Nobel-prize-winning IBM invention that uses the tip of a sharp metal needle to scan a surface to image and move individual atoms with great precision.
Three years ago, our group showed that we could detect the magnetism of the electrons of a single atom and use its sensitivity to magnetic fields as a way to detect and measure the properties of nearby magnetic atoms on the surface. Now, we have extended this to detect the much tinier magnetism of the nucleus.
Figure 2: Scanning tunneling microscope image of the magnesium oxide surface, where the small protrusions are individual iron atoms.
(Image courtesy QNS)
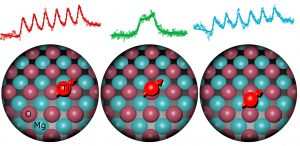
The interaction between an atom’s nucleus and its electrons, called the hyperfine interaction, makes it possible to detect the magnetism of the nucleus. The hyperfine interaction within each atom changed when we moved the atom to a different position or if we moved another atom close to it. We used the STM to reposition individual atoms and show that the hyperfine interaction depends strongly on chemical bonding to other atoms. For example, a titanium atom bonded to four nearby atoms yielded a much stronger hyperfine interaction than the same titanium atom perched atop a single oxygen atom. Furthermore, we found that the strength of the hyperfine interaction depends on the presence of neighboring magnetic atoms, so it reveals how the magnetism of the two atoms combine according to the rules of quantum mechanics.
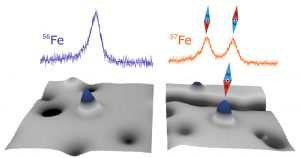
Figure 3: Two iron atoms, seen as blue hills in the lower images, having different isotopes. The right atom is the isotope iron-57, which has a nuclear spin. As a result, two peaks are observed in its energy spectrum, corresponding to the two possible orientations for the spin of the nucleus.
(Image courtesy QNS)
The nucleus of an atom is made of protons and neutrons, and the number of protons determines which element the atom is. The magnetism of the nucleus comes from a property called “spin” because it behaves much like a spinning ball of electric charge. Only some isotopes have a nucleus with spin, and this spin makes a small magnetic field, just as the earth has a magnetic field due to the electric charge that circulates deep in its core. The magnetic field from the nuclear spin is so miniscule that it is difficult to detect, except when many millions are measured at the same time. This is the basis for medical MRI imaging machine, which measures many trillions of nuclear spins for each point in the resulting images.
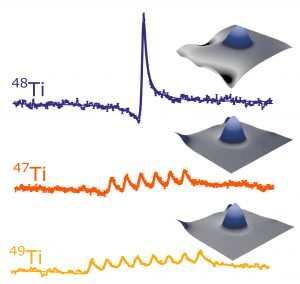
Figure 4: Energy spectra measured on individual titanium atoms. Two isotopes have a high nuclear spin and thus display multiple peaks, one peak for each orientation of the nucleus.
(Image courtesy QNS)
To detect the spin of a single nucleus, we make use of the electrons surrounding the nucleus – together the nucleus and the electrons form an atom. These electrons also have spin. For electrons, the spin results in a magnetic field that is about a thousand times larger than for the nucleus. This makes detecting the electrons much easier, but still exceedingly challenging to sense for a single atom at a time.
We use an advanced form of scanning tunneling microscopy that operates in ultra-cold, clean, vibration-free conditions to ensure that the atoms stay in place and the sensitive measurements are not disturbed by heat, debris or noise.
Our team detects a single atom’s spin by using an ultra-sensitive technique called spin resonance in which we use the tip of the STM to find and select a single atom to examine. Then we use electron spin resonance (ESR), which senses how fast the north pole of the electron rotates. This rotation is called precession, and the north pole moves much like the axis of a spinning top placed on a table, which slowly rotates to point in different directions. For an electron, precession happens billions of times per second, and the frequency of precession is called the resonant frequency. This frequency changes in response to subtle changes in the magnetic field experienced by the atom. Performing ESR using a scanning tunneling microscope allows us to measure the spin while seeing the position of the atom and those it is bonded to, along with the more distant atoms that subtly influence it, revealing invaluable information about the magnetic interaction at the single-atom scale, which is essential for designing advanced electronic devices made from several atoms.
Figure 5: A single titanium atom is moved to three different positions on the surface. This changes the spectrum, because the interaction with the nuclear spin is sensitive to the chemistry of the binding site.
(Image courtesy QNS)
Using spin resonance, a single atom serves a sensitive probe of the magnetic field, right at the position of the atom. Our team previously used this to detect the magnetic field of nearby atoms placed on the surface. In these studies we used iron and titanium atoms, which each have unique properties. We even found that individual atoms of the element holmium act as tiny permanent magnets so they can store information. These advances all led up to our latest breakthrough, in which we detect the magnetism of nucleus and the information that it reveals.
Paper: Hyperfine interaction of individual atoms on a surface
Science has published an article written by Philip Willke, Yujeong Bae, IBM Almaden Research Center, San Jose, CA 95120, USA, Center for Quantum Nanoscience, Institute for Basic Science (IBS), Seoul 03760, Republic of Korea, and Department of Physics, Ewha Womans University, Seoul 03760, Republic of Korea, Kai Yang, IBM Almaden Research Center, San Jose, CA 95120, USA, Jose L. Lado, QuantaLab, International Iberian Nanotechnology Laboratory (INL), 4715-310 Braga, Portugal, and Institute for Theoretical Physics, ETH Zurich, 8093 Zurich, Switzerland, Alejandro Ferrón, Instituto de Modelado e Innovación Tecnológica (CONICET-UNNE), Facultad de Ciencias Exactas, Naturales y Agrimensura, Universidad Nacional del Nordeste, W3404AAS Corrientes, Argentina, Taeyoung Choi, Center for Quantum Nanoscience, Institute for Basic Science (IBS), Seoul 03760, Republic of Korea, and Department of Physics, Ewha Womans University, Seoul 03760, Republic of Korea, Arzhang Ardavan, Centre for Advanced Electron Spin Resonance, Clarendon Laboratory, Department of Physics, University of Oxford, Oxford OX1 3PU, UK, Joaquín Fernández-Rossier, QuantaLab, International Iberian Nanotechnology Laboratory (INL), 4715-310 Braga, Portugal, Andreas J. Heinrich, Center for Quantum Nanoscience, Institute for Basic Science (IBS), Seoul 03760, Republic of Korea, and Department of Physics, Ewha Womans University, Seoul 03760, Republic of Korea, and Christopher P. Lutz, IBM Almaden Research Center, San Jose, CA 95120, USA.
Abstract: “Taking advantage of nuclear spins for electronic structure analysis, magnetic resonance imaging, and quantum devices hinges on knowledge and control of the surrounding atomic-scale environment. We measured and manipulated the hyperfine interaction of individual iron and titanium atoms placed on a magnesium oxide surface by using spin-polarized scanning tunneling microscopy in combination with single-atom electron spin resonance. Using atom manipulation to move single atoms, we found that the hyperfine interaction strongly depended on the binding configuration of the atom. We could extract atom- and position-dependent information about the electronic ground state, the state mixing with neighboring atoms, and properties of the nuclear spin. Thus, the hyperfine spectrum becomes a powerful probe of the chemical environment of individual atoms and nanostructures.“















 Subscribe to our free daily newsletter
Subscribe to our free daily newsletter

